News and Award
About CMUH
China Medical University Hospital Pioneers the Design of CAR.BiTE-GDT Cell Therapy. Over 90% of Solid Cancer Cells Are Eradicated by Allogeneic Multi-Target nano-CAR-T in Animal Experiments.
Cancer has long been one of the leading causes of death not only in Taiwan but also worldwide. Cell therapy is a developing approach for treating cancer and other diseases that involves the administration of specific cells, such as immune cells or stem cells. However, solid tumors (such as lung cancer, breast cancer, and colorectal cancer, etc.) are still regarded as the most difficult field to conquer, despite accounting for more than 90% of all cancer patients. Professor Cho Der-Yang, the Superintendent of the China Medical University Hospital, and the Director of the Translational Cell Therapy Center (third from the right in the front row), led the research team and has achieved a significant global breakthrough. Animal experiments have yielded remarkable results after years of research and development of “allotransplantation, non-viral driven multi-target nanobody-based CAR.BiTE-gdT cell therapy”. These results include: effectively penetrate into solid cancer and secrete BiTE to engage and activate peripheral immune cells to jointly fight cancer cells; three- to five-fold greater survival in mouse models of lung cancer and triple-negative breast cancer, while also eliminating more than 90% of cancer cells; this novel type of nanobody-based CAR.BiTE engineered gdT cells can destroy cancer cells with antigenic heterogeneity due to mutation or loss of the target.
In March of 2023, the internationally renowned journal ‘Advanced Science’ accepted the publication of a number of innovative biotechnology research findings from the China Medical University Hospital (Adv Sci (Weinh). 2023 Apr 20;e2206856. doi: 10.1002/advs.202206856.). The relevant research technology has been reviewed for patentability in the United States and other countries, and has completed the technology transfer to Ever Supreme Bio Technology Co., Ltd. for process development and clinical trial application filing.
“CAR.BiTE-T cell therapy”, developed by the China Medical University Hospital, as noted by Superintendent Cho Der-Yang, is focused mainly on solid cancer patients and has made significant contributions to global medicine. After years of research and solving difficulties in the laboratory, the expert team has successfully overcome the insurmountable barriers in the existing cell theory, making it possible to fight against a variety of solid tumors (lung cancer, colorectal cancer, breast cancer). Allograft products are readily available (obtained and used) in large quantities and at scale, , saving cancer patients the time and risks of waiting for autologous cell production. In addition, “CAR.BiTE-T cell therapy” significantly lessens production costs, which is conducive to wide application. It could even be used to treat cancer patients with varying levels of HLA-G expression. It is expected to significantly improve patient prognosis and prolong survival time in combination with targeted therapy and chemotherapy.
CAR.BiTE-gdT is suitable for allogeneic transplantation, not driven by genetically modified viruses, and easily available on shelves without waiting.
According to Superintendent Cho Der-Yang, “The six existing CAR-T products or even CAR-T products under development are all genetically modified using parent’s autologous immune cells, and then reinfused back into the patient’s body to fight cancer.” For terminal cancer patients, having to wait two months cell products are too late. In addition, because the immune system is already compromised, cancer patients often have low quality immune cells to produce cellular products. Furthermore, the bloodstream of advanced cancer patients contains metabolic cancer cells, which can easily contaminate the autologous CAR-T products. Another major concern with current CAR-T treatments for cancer patients is the mutation risk associated with using viruses to geneticlly engineer CAR-T cells.
Superintendent Cho Der-Yang stressed that in response to the above clinical plights, the expert team of China Medical University Hospital has developed a multi-target, multi-functional CAR.BiTE-gdT, which is an immune cell therapy program of “allogeneic transplantable, non-viral driven”. What's more valuable is that the team of experts has designed a strategy of “grab-and-go” use compared to general medicines on the shelf. For the rescue of terminal cancer patients, it is a race against time and solves the problems of the quality of the patient's autoimmune cells and the additional risk of cancer.
Four Key Achievements of CAR.BiTE-gdT Reverse Immunosuppression to Activate Immune Cells, and Are Still Effective for Antigen Loss.
The biggest breakthrough in immune cell therapy in recent years is the genetically modified immune cell therapy CAR-T cells approved by the FDA in the United States. Genetically modified T cells cultured in large quantities in vitro and reinfused into patients can recognize cancer cells and attack them, achieving great success in the treatment of blood cancers. However, currently there are no CAR-T cell products that can be clinically used for solid cancers (such as lung cancer, colorectal cancer and breast cancer ) that account for the majority of cancer patients (90%). Unlike blood cancer cells, solid cancer cells have a castle-like structure that makes it difficult for immune cells to infiltrate. They also express many immune interfering factors such as HLA-G (about 30% expression rate, the higher the degree of malignancy, the more) and PD-L1 (about 1 to 40% expression rate) to suppress and deplete immune cells. In addition, solid cancer cells might relapse rapidly after single-target CAR-T therapy due to the heterogeneity of antigens, which prevents single-target CAR-T cells from effectively eradicating cancer cells. Therefore, multi-targeting, multi-functionality, and the ability to prevent immunosuppression should be the key to the success of CAR-T in the treatment of solid cancers. However, there are currently no CAR-T products approved for the treatment of solid cancers in the world, and only a few dual-targeted CAR-T products are undergoing clinical trials.
CAR.BiTE-gdT has demonstrated at least four key effects in animal experiments, as further explained by Superintendent Cho Der-Yang of the China Medical University Hospital:
- Effectively penetrate into solid cancers and secrete BiTE (dual targeting T-cell activating antibody) to activate peripheral immune cells to jointly fight against cancer cells.
- 2. In addition to complying with the US FDA allogeneic transplantation regulations, the cell prototype product does not harm normal tissue cells expressing CAR.BiTE targets.
- The multi-target and multi-functional CAR.BiTE-gdT has been shown to effectively prolong the survival time by three- to five-fold, and even the cancer cells almost disappeared for a period of time..
- The gdT with natural anti-cancer ability carry the novel nanobody-based CAR.BiTE, even if the cancer target is mutated or lost, and the cancer cells with antigen heterogeneity cannot escape.
“Allogeneic transplantable non-viral gene-modified multi-targeted nanobody CAR.BiTE-gdT immune cell therapy” demonstrates the following five benefits in terms of patient treatment and manufacturing processes:
- CAR.BiTE-gdT has the potential to fight against a variety of refractory solid cancers.
At present, there are no CAR-T cell products that can be clinically used for solid cancers, which account for the majority of cancer patients. The expert team of the China Medical University Hospital has proved that the multi-targeted and multifunctional CAR.BiTE-gdT kills more than 90% of solid cancer cells in lung cancer and triple-negative breast cancer mouse models, and effectively prolongs the survival time by 3-5 times.
- . It can solve the risk that cancer patients spend time waiting for the manufacture of autologous cell product.
- The allogenic CAR.BiTE-gdT cells can be used immediately on the day when the patient's tests is completed. Compared with autologous CAR-T, which takes at least one and a half months to prepare, it has more time advantages to rescue advanced cancer patients and solve the problems of the quality of patients' autoimmune cells and additional cancer-causing risks.Allogeneic cell products can be prepared in large quantities and have the characteristics of taking and using at any time.
After allogeneic CAR.BiTE-gdT is prepared, frozen and inspected, it can be thawed and used at any time according to the patient's needs, and a therapy session can be completed within half a day.
- Lessen production costs drastically.
At present, the cost of CAR-T cell therapy is about 12-15 million NT dollars, wherease CAR.BiTE-gdT is expected to be reduced to about 1/5 of the cost compared with autologous CAR-T under the automated large scale production process. Low prices will increase the chances of widespread adoption of cell therapy.
- Significantly improves patient prognosis and prolongs survival time.
Can be applied to patients with HLA-G-expressing cancers, and can be combined with targeted therapy and chemotherapy to prolong survival time..
Six major patents granted by the United States, Japan, the European Union, and Taiwan
The research paper "BiTE-secreting CAR-γδT as a dual targeting strategy for the treatment of solid tumors" by China Medical University Hospital was published in the authoritative international journal "Advanced Science" in April this year. It has been highly concerned by cell therapy scholars and experts in the field of biotechnology around the world. Relevant patents have granted or are undergoing patent reviewing in the United States and other countries. Relevant technologies have been transferred to Ever Supreme Bio Technology Co., Ltd. for product manufactural conversion and clinical trial preparation. Clinical trials are expected to be carried out within this year, and applied to lung cancer, colorectal cancer, and triple-negative breast cancer patients with HLA-G targets. This brings new strategies for the treatment of advanced solid cancers.
The China Medical University Hospital’s expert team, led by Superintendent Cho Der-Yang, has developed an allogeneic transplantable, message RNA (mRNA) engineering, multi-targeted, and nanobody-based CAR.BiTE-gdT cell therapy that can be used to treat a variety of refractory solid cancers ( Figure 1), HLA-G and PD-L1 are often co-expressed in patient cancer tissues (Figure 2), CAR.BiTE-gdT cells effectively penetrate into solid cancer tissues (Figure 3), and further eliminate cancer cells (Figure 4) and activate other immune cells to jointly fight against cancer cells (Figure 5) without harming normal tissues (Figure 6).
.png)
Figure 1
Conventional CAR-T cell development is limited. Conventional CAR-T cells rely on the preparation of the patient's own immune cells. This personalized CAR-T cell manufacturing process is time-consuming (more than one month) and expensive (NT$10-15 million). The quality of CAR-T cells is often unstable due to the poor state of immune cells in cancer patients. In patients with solid cancers (such as lung cancer and triple-negative breast cancer), the heterogeneity of tumor antigens will make conventional single-target CAR-T unable to eliminate all solid cancer cells. And solid cancer cells will express many immunosuppressive molecules such as HLA-G and PD-L1 to deplete the activity of CAR-T cells so that they cannot kill cancer cells. Furthermore, the conventional CAR-T process relies on viral vectors for genetic modification, which has the risk of mutation or even carcinogenicity.
.png)
Figure 2:
The China Medical University’s CAR.BiTE-gdT cell product technology is leading and innovative.. The multi-targeted CAR.BiTE-gdT cells feature the following to overcome the limitations of conventional CAR-T cells: 1. Easily accessible (take and use) without waiting, which can solve the time-consuming process of cancer patients who cannot wait for autologous cells because of the rapid course of disease.; 2. The multi-target design can effectively eradicate solid cancer cells by overcoming antigen heterogeneity; 3. Genetic engineering with messenger RNA (mRNA) can avoid mutations; 4. Automated large-scale production capacity to prepare CAR.BiTE-gdT cells with stable quality; 5. Compared with the antibody molecules used in traditional CAR, the whole nano antibody CAR-T has high affinity, specificity and tissue penetration, and can more effectively identify tumor antigens; and 6. Different from conventional CAR-transfected T cells, the China Medical University Hospital uses inherently multifunctional for genetic engineering. gdT cells are naturally capable of regulating the immune system, tolerating allotransplantation without rejecting the host, and exhibiting anticancer properties. In addition to the instantaneous availability of the China Medical University Hospital’s nanobody-based CAR BiTE-gdT cells for allotransplantation, the immunosuppression and antigen heterogeneity caused by the immunosuppressant molecules of solid cancers can be overcome.
.jpg)
Figure 3:
The Mechanism of Action of CAR001: Based on the properties of CAR.BiTE-gd(GDT), it can completely identify heterogeneous tumor antigens and reduce tumor immunosuppression.
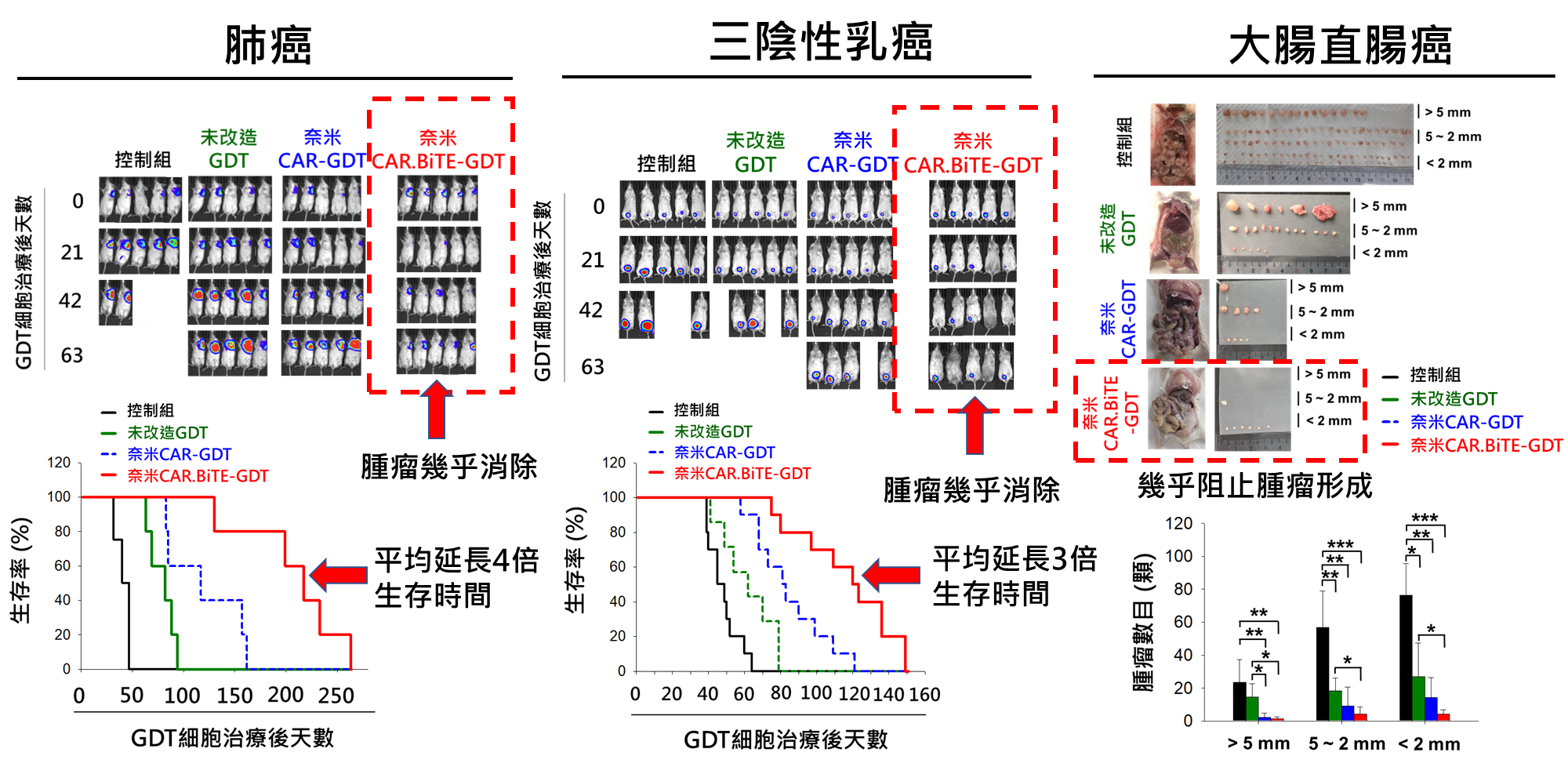
Figure 4:
In experimental mouse models, CAR.BiTE-gdT (GDT) cells are effective in treating lung cancer, breast cancer, and colorectal cancer. CAR.BiTE-gdT (GDT) cells can, on average, prolong the average survival time by 4-times in mice with lung cancer (left)and 3-times in triple-negative breast cancer (middle), as well as significantly inhibit tumor formation in mice with colorectal cancer (right).
.jpg)
Figure 5:
A comparison chart of CAR.BiTE-gd T cell technology (CMUH CAR001) for allotransplantation at the China Medical University Hospital and its global counterparts, CAR-T, mRNA CAR-T, and multi-target CAR-T. Only CMUH CAR001 combines the use of V2 (V delta 2) T cells, allotransplantation, dual-target and targeted immune checkpoint, BiTE antibody secretion, mRNA driven genetic modification, and application in a variety of solid cancers, according to comparisons made between similar current CAR-T technologies. It is currently undergoing pre-clinical testing, and a submission for US IND new drug development review is scheduled for mid-June to July this year.
.jpg)
Figure 6: Superintendent Prof. Cho Der-Yang of the China Medical University Hospital and the Director of the Translational Cell Therapy Center (third from the right in the front row) led the research team and achieved a major global breakthrough. After years of research and development of “allogeneic transplantable” and “non-virus-driven genetic engineering multi-targeted nanobody-based” CAR.BiTE-T immune cell therapy has shown remarkable results in animal experiments, including penetrating into solid cancers effectively and secreting BiTE (Bi-directed T-cell activating antibody) to activate peripheral immune cells and jointly fight against cancer cells. The relevant research technology has been patented in the United States and other countries, and the technology has been transferred to Ever Supreme Bio Technology Co., Ltd. Chairman Liu Chu-Chi (second from the right in the front row) and General Manager Huang Wen-Liang (fourth from the right in the front row) jointly attended the event and declared the beginning of process development and clinical trial application filing.
.jpg)
Figure 7: Superintendent Cho Der-Yang stated that the current six CAR-T products and the CAR-T products in development are genetically modified using the patient’s autologous immune cells, which are then reinfused into the patient’s body to fight against cancer. For patients with terminal cancer, having to wait for more than one month for the production of cell products is a slow and inadequate response to an emergency. The China Medical University Hospital’s CAR.BiTE-gdT-cell technology combines the use of allogeneic transplantable, dual-targeted against immune checkpoints, BiTE antibody secretion, mRNA-driven genetic modification, and application in a range of solid cancers. It is currently undergoing pre-clinical testing, and a submission for US IND new drug development review is scheduled for mid-June to July this year.
.jpg)
Figure 8: As pointed out by Ever Supreme Bio Technology Co., Ltd. Chairman Liu Chu-Chi, the price of CAR-T cell therapy is around NT$12-15 million, whereas the cost of the China Medical University Hospital’s CAR.BiTE-gdT, under automated mass preparation processes, can be reduced by five times compared to autologous CAR-T culture process costs, making cell therapy more affordable and accessible to a larger patient population.
.jpg)
Figure 9:
Superintendent Cho Der-Yang of the China Medical University Hospital (CMUH) said that the CMUH CAR.BiTE-gdT cells are at the forefront of a number of technological advancements. 1. Easily accessible (take and use) without waiting; 2. The multi-target design can successfully eradicate solid cancer cells by overcoming antigen heterogeneity; 3. RNA (mRNA)-driven genetic engineering can prevent mutations; 4. Automated mass production leads to the preparation of large quantities of high-quality CAR.BiTE-gdT cells; 5. Complete nanobody transformation; and 6. Use of the naturally multipotent gdT cells. In addition to its anti-cancer properties, gdT has the innate ability to regulate the immune system, which allows for allotransplantation without host rejection.
.jpg)
Figure 10: The China Medical University Hospital’s research paper was accepted and published in the prestigious international journal “Advanced Science” in the field of scientific research this year (Adv Sci (Weinh). 2023 Apr 20;e2206856. doi: 10.1002/advs.202206856.) and has since attracted the attention of cell therapy scholars and biotechnology specialists from around the world. Patentability is being reviewed in the United States and numerous other countries, and the transfer of technology to Ever Supreme Bio Technology Co., Ltd. has been completed in preparation for product process conversion and clinical trial preparation. Within this year, it is anticipated that clinical trials will be conducted to treat cancer patients with tumor antigen HLA-G expression in lung cancer, colorectal cancer, and triple-negative breast cancer. This may lead to a new strategy for the treatment of terminal-stage solid cancers.



